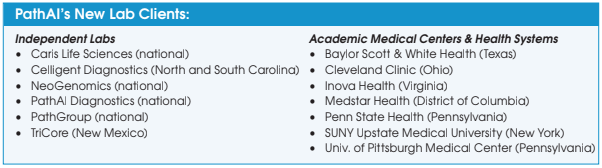
Versant Diagnostics and In-Office Pathology Team Up
Versant Diagnostics and In-Office Pathology Team Up
Versant Diagnostics (Grapevine, TX) has signed an agreement with In-Office Pathology LLC (Nantucket, MA). Versant will offer professional pathology services to IOP’s network of in-office labs, and IOP will build histology labs at select Versant Diagnostics specialty clinics and physician practices.
IOP was founded by Joe Plandowski and Bernie Ness in 2004. Over the past 18 years, IOP has developed in-office histology labs at 99 specialty groups in 30 states. The majority of these labs are located at gastroenterology practices and others are located at urology, dermatology and podiatry groups.
Under the new agreement, IOP’s lab clients will continue to directly bill payers for global lab services, including professional and technical fees. Versant’s pathologists will provide professional interpretations at a negotiated fee. Initially, Versant is expected to provide professional services to IOP’s lab clients in the greater Chicago area. In the future, new and existing IOP lab clients from around the country will have the option of installing slide scanners so that Versant’s pathologists can provide professional interpretations from digital images.
Plandowski says that finding and hiring pathologists has become one of IOP’s biggest challenges. He notes that after a pandemic slowdown in 2020-2021, IOP has since signed seven new clients, including three gastroenterology groups, three podiatry groups and one derm group.
Versant is a startup pathology company founded by Ven Aduana, MD, Jim Billington and Brian Carr in 2021 (see LE November 2021). Versant, which currently has 15 pathologists, has $100 million in financing (both equity and debt) from Iron Path Capital (Nashville, TN). Versant is acquiring pathology practices and then seeking to add volume through the use of digital pathology.