Castle Biosciences To Buy AltheaDx For Up To $140 Million
Castle Biosciences (Friendswood, TX) has agreed to acquire AltheaDx (San Diego, CA) for $65 million in initial consideration consisting of $32.5 million in cash plus $32.5 million in stock. In addition, Castle could pay up to $75 million more in cash and stock if AltheaDx hits certain revenue targets over the next three years and gets expanded Medicare coverage for its IDgenetix
test. The deal is expected to close this summer.
AltheaDx markets a laboratory-developed pharmacogenomic test under the brand name IDgenetix. The cheek-swab test analyzes a panel of 15 genes to help doctors make prescription recommendations for patients with depression. The test is performed at AltheaDx’s CAP-accredited laboratory in San Diego. The Medicare program has covered IDgenetix since the fall of 2020 at a rate of $1,569 (CPT 81479: unlisted molecular pathology procedure).
AltheaDx, which has 40 employees, generated revenues of less than $1 million in 2021. Castle anticipates that AltheaDx will record $1-3 million of revenue in 2022.
Privately-held AltheaDx’s largest investors include Alma Life Sciences, Ally Bridge Group and WuXi Healthcare Ventures. AltheaDx filed for an initial public stock offering in December 2014, but shelved the proposed stock sale in early 2015.
Castle, which has 345 employees, is headquartered in Friendswood, Texas (near Houston) and operates CLIA-certified labs in Phoenix and Pittsburgh. Its lead testing product is DecisionDxMelanoma, which analyzes 31 genes to predict metastatic risk in patients diagnosed with cutaneous (skin) melanoma. DecisionDx-Melanoma is an Advanced Diagnostic Laboratory Test (ADLT) that is reimbursed by Medicare at $7,193 (CPT 81529).
For the full year ended December 31, 2021, Castle reported a net loss of $31.3 million versus a net loss of $10.3 million in 2020; revenue increased by 50% to $94.1 million.
High Claims Denials for Pharmacogenomic Testing
To date, pharmacogenomic testing for medication selection has been a tough market for labs providing this service. The volume of allowed Medicare Part B carrier claims for four key codes used to bill for pharmacogenomic 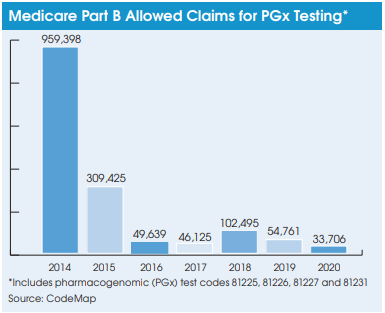 testing (aka cytochrome p450; CPT 81225, 81226, 81227 and 81231) declined from a combined total of 959,398 allowed claims in 2014 to 33,706 allowed claims in 2020, according to data from the coding and reimbursement firm CodeMap (Chicago, IL). Furthermore, CodeMap data show that denial rates for these pharmacogenomic testing codes ranged from an average of 26% (2014) to 85% (2019) over the seven-year period. This compares with average claims denials rates of less than 10% for most routine clinical lab tests.
testing (aka cytochrome p450; CPT 81225, 81226, 81227 and 81231) declined from a combined total of 959,398 allowed claims in 2014 to 33,706 allowed claims in 2020, according to data from the coding and reimbursement firm CodeMap (Chicago, IL). Furthermore, CodeMap data show that denial rates for these pharmacogenomic testing codes ranged from an average of 26% (2014) to 85% (2019) over the seven-year period. This compares with average claims denials rates of less than 10% for most routine clinical lab tests.