ACLA Wins Appeals Court Decision; HHS Likely To Request Rehearing
ACLA Wins Appeals Court Decision; HHS Likely To Request Rehearing
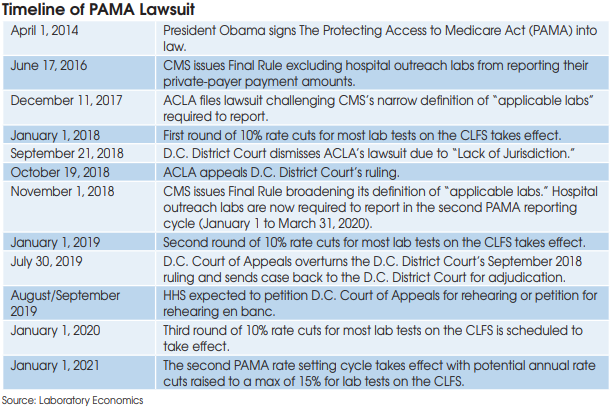
On July 30, the U.S. Court of Appeals for the District of Columbia ruled in favor of the American Clinical Laboratory Assn. (ACLA) in its suit against the U.S. Department of Health and Human Services (HHS) regarding implementation of the Protecting Access to Medicare Act (PAMA). The ruling overturned a D.C. District Court decision that dismissed ACLA’s lawsuit on the grounds that PAMA law prohibits judicial review of CMS’s “establishment of payment amounts” for clinical lab tests.
However, before the case goes back to D.C. District Court for review on its
merits, HHS is likely to request either a rehearing of the D.C. Court of Appeals’ three-judge panel decision or petition for a rehearing en banc from a broader panel of judges.
The D.C. Court of Appeals concluded that the statutory provision stripping jurisdiction to review payment amounts does not cover the statute’s data-collection provision. It ruled that the case be sent back to D.C. District Court to determine whether or not HHS/CMS violated PAMA by excluding hospital outreach labs from the first private-payer payment survey used to set Medicare CLFS rates for 2018 to 2020.
HHS has 45 days to petition for either a rehearing or rehearing en banc. The D.C. Court of Appeals has ordered that the case be held from being sent back to the D.C. District Court “until seven days after disposition of any timely request for rehearing or petition for rehearing en banc.”
It’s unlikely that any request by HHS for a rehearing will be granted, but the process will delay final resolution of the lawsuit, which is now approaching two years since initially being filed in late 2017.
More likely than not, the D.C. Court of Appeals will uphold its initial decision and send the lawsuit back to D.C. District Court. But the D.C. District Court proceedings could be lengthy, taking anywhere from six months to more than two years, before a decision is rendered.
Nonetheless, the Appeals Court decision is a victory for ACLA that will put pressure on HHS/CMS to negotiate with ACLA for a potential settlement of the lawsuit. ACLA and its biggest members, LabCorp and Quest Diagnostics, are lobbying hard to delay the next private-payer payment data reporting period (currently scheduled for January 1 to March 31, 2020) by one year. In addition, the lab industry wants CMS to use statistical sampling when it calculates new CLFS rates for 2021 to ensure that all lab segments (independents, hospital outreach labs and POLs) are properly represented.