Monkeypox Cases May Be Vastly Undercounted
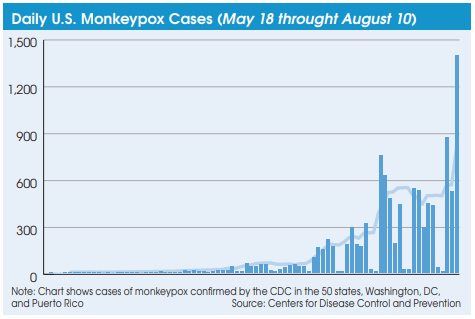
Based on CDC case counts, the current monkeypox outbreak in the U.S. doesn’t look that bad. As of August 10, the CDC reports a total of 11,177 Monkeypox cases had been recorded since the initial case was identified in Massachusetts on May 18, 2022.
However, the official case count is misleading, according to Manoj Gandhi, MD, PhD, Senior Medical Director at Thermo Fisher Scientific. Gandhi thinks the number of unreported cases is bigger — probably much bigger — than the CDC case count suggests. “We’re just seeing the tip of the iceberg,” says Gandhi. The CDC is currently only reporting monkeypox cases on a weekly basis. The next case count will be announced on August 17.
Thermo Fisher is manufacturing reagents that labs can use to develop their own laboratory-developed Monkeypox tests to run on QuantStudio 12K Flex PCR systems. LDT testing will augment the Monkeypox testing that is being performed by labs with access to CDC-developed kits, which are in limited supply.
Currently, the CDC-developed kits for Monkeypox testing are being used by 78 public health labs and five chosen commercial labs, including Aegis Sciences, Labcorp, Mayo Clinic Labs, Quest Diagnostics and Sonic Healthcare. Total combined capacity for public health and the five commercial labs is more than 60,000 Monkeypox tests per week.
In late July, the American Medical Association (AMA) issued a CPT code (87593) for molecular diagnostic testing that detects the monkeypox virus. A Medicare reimbursement rate has not been established yet for CPT 87593 and it is currently in the MolDx program for pricing, according to Lale White, Executive Chairman and CEO at XIFIN Inc. “Labs need to know Monkeypox tests are reimbursable, so they are encouraged to test,” notes Thermo’s Gandhi.
Test samples for Monkeypox are collected by swab from a patient’s blister. People testing positive can be treated with the prescription drug tecovirimat, also called Tpoxx, which is in short supply. Most importantly, Gandhi says positive cases should isolate to prevent the spread. And family
members and close contacts should get tested.
Monkeypox is usually a self-limited disease with the symptoms lasting from 2 to 4 weeks, according to the World Health Organization. However, severe cases can occur. There have been no reported deaths from Monkeypox to date in the United States.
Mako Medical Labs (Raleigh, NC) is one of the first independent labs to offer an LDT for Monkeypox. Mako went live with Monkeypox testing on July 18 on Thermo’s QuantStudio, according to Matthew Tugwell, Director, Genomics at Mako.
Tugwell says Mako currently has the capacity to perform up to 30,000 Monkeypox tests per day. The majority of Mako’s samples for monkeypox testing are coming from STD clinics. Patients getting tested for sexually transmitted diseases like herpes and syphilis are also being tested for
Monkeypox. Positivity rates are currently averaging about 13%. So far, Tugwell says demand for Monkeypox testing has been low—probably because people are shying away from getting tested because of a perceived
stigma associated with the disease.
So far, nearly all Monkeypox cases in the United States have been among gay and bisexual men. However, Tugwell notes, “We don’t really know where this is going. It has the potential to infect anyone, regardless of gender, skin color or sexual preferences.”