FDA Won’t Extend Comment Period on LDT Regs
FDA Won’t Extend Comment Period on LDT Regs
The FDA has announced that its standard 60-day comment period for its proposed regulation of laboratory-developed test (LDTs) will not be extended past December 4. Several trade groups, including CAP and ACLA, had requested to stretch the comment period to 120 days. An extension would have potentially delayed implementation of the rule while giving Congress more time to pass LDT reform legislation. The FDA has indicated that a final rule could be published as early as April 1, 2024.
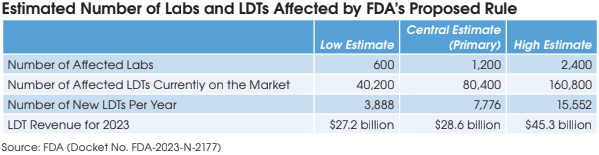
The FDA estimates that there are approximately 12,000 U.S. labs performing high-complexity testing and that 10% of these are marketing LDTs. These 1,200 labs are currently offering 80,400 LDTs and 7,776 new LDTs are coming to the market each year. The FDA estimates that labs will generate $28.6 billion of revenue from LDTs in 2023.
One of the biggest concerns is the FDA’s capacity to review and process as many as 80,000+ LDT applications over the next few years. For example, the FDA gave marketing authorization to a grand total of only 765 medical devices, including lab tests, in 2022, according to the FDA’s Center for Devices and Radiological Health 2022 Annual Report. The FDA’s ability to process tens of thousands of LDT applications in a timely manner, even with the help of third-party reviewers, is doubtful.
During an October 24 call with investors, Jim Davis, Chief Executive of Quest Diagnostics, said that Quest agrees with the long-standing assertion of the American Clinical Laboratory Assn. (ACLA), “that the FDA does not have the statutory authority to unilaterally regulate LDTs under its existing medical device authority.” Davis noted that by volume, LDTs account for less than 10% of Quest’s overall testing business. Quest performs the majority of its LDT testing at three ISO-certified labs, including San Juan Capistrano, CA; Chantilly, VA; and Lewisville, TX.
On an October 26 conference call, Labcorp CEO Adam Schechter said that LDTs account for less than 5% of Labcorp’s volume and less than 10% of its testing revenue. “If you look at the rigor that we go through with our laboratory-developed tests, we think we do the vast majority of what they [FDA] would be asking for anyway.” Schechter said FDA regulation could have unintended consequences. LDTs “are sometimes the most important test for new specialty areas. And getting those tests to patients quickly is what’s most important.”
One group in favor of LDT regulation is AdvaMed (Washington, DC), whose members include Abbott, BD, Bio-Rad Laboratories, bioMerieux, Hologic, Illumina and Roche. “We like the rule….This is really about making sure there’s a level playing field when you’re talking about diagnostics tests and having everything go through a similar pathway,” said AdvaMed CEO Scott Whitaker at an October 10 press conference. “Either you raise the regulatory standard, or you lower the regulatory standard, but you do it across the board without stifling innovation,” he said.
The last major effort by the FDA to formally regulate LDTs came in October 2014, when the agency issued draft guidance. But after collecting comments and facing two years of stiff opposition, the agency chose not to issue final guidance. One of the strongest critics was ACLA, which challenged the FDA’s authority over the tests by filing a citizen petition and making clear its intent to sue if necessary.