AMA Announces New Add-On Digital Pathology Codes
AMA Announces New Add-On Digital Pathology Codes
The American Medical Association (AMA) CPT Editorial Panel has
announced 13 new digital pathology add-on codes effective on January
1, 2023. The new digital pathology Category III CPT codes will be used
to report additional clinical staff work and service requirements associated
with digitizing glass slides for primary diagnosis.
Introduction of the codes will allow CMS to monitor the usage of digital
pathology. However, no relative value units (RVUs) or national payment
rates have been assigned to the new codes.
“It’s an important first step, but widespread use in clinical practice will
need to be demonstrated before the new codes are moved to Category I
and assigned RVUs,” notes Jonathan Myles, MD, Chair of the Council
on Government and Professional Affairs at the College of American
Pathologists (CAP).
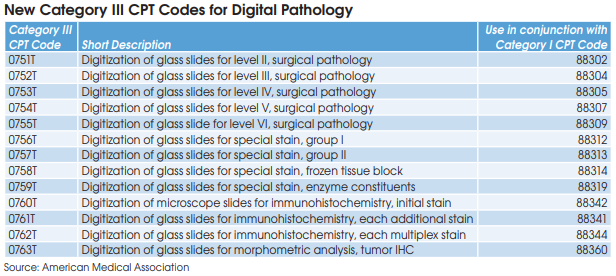
The new digital pathology add-on codes are linked with 13 of the most commonly billed pathology procedures, including CPT 88305 (Level IV-Tissue Exam). CAP’s Myles says that the new add-on codes should only be reported when used for clinical diagnosis and not for things like archiving slides, training or validation of AI algorithms, or tumor board conferences. “It’s clear that digital pathology will be a part of the practice of pathology and lab medicine, but it has to be proven to be in widespread clinical use to gain Medicare reimbursement,” says Myles.
Assuming that digital pathology volumes prove to be significant, the very earliest that CMS could assign RVUs and establish national payment rates for the new add-on codes would be for an effective date of January 1, 2024, notes Laboratory Economics. However, the process is more likely to take at
least a few years. In the meantime, each individual Medicare Administrative Contractor (MAC), as well as private insurers, could establish their own payment rates, but are not required to do so.
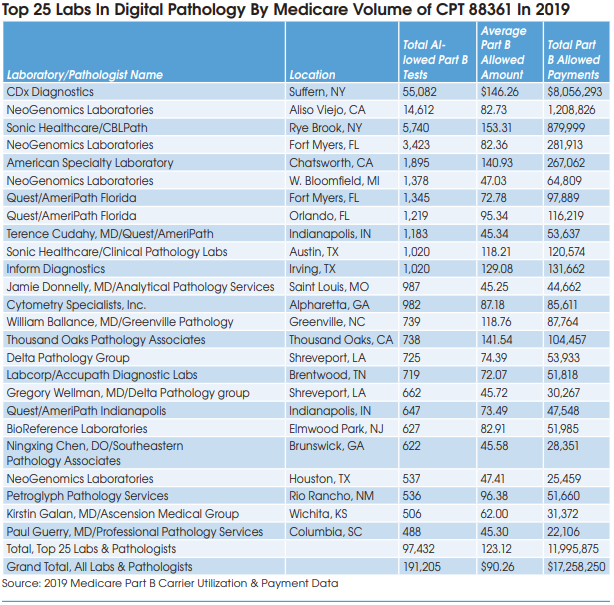
Many pathology labs in the United States are experimenting, but very few have gone fully digital, according to Michael Rivers, Vice President and Lifecycle Leader for Digital Pathology, Roche Tissue Diagnostics (Santa Clara, CA). “Digitization is a means to an end. It will allow the application
of innovative AI solutions to pathology images and ultimately integrated multi-modal analysis of patient cases combining anatomic pathology, clinical lab and gene-sequencing data,” says Rivers.
“My prediction is that if the Category III codes are converted to Category I code status, in the future Medicare could potentially reimburse the new add-on codes at roughly 3% to 5% of the global rates for related existing codes,” says Erick Lin, MD, PhD, Senior Director, Medical Affairs, PathAI (Boston, MA). Thus, the add-on code for digitizing one unit of CPT 88305 (current global rate of $72) could be reimbursed at between $2 and $4. The key is for all pathology labs to be aware of the new add-on codes, prepare systems to report, and then begin reporting the new codes effective January 1, 2023. If all clinical utilization is appropriately reported on claims, it can help facilitate Medicare’s establishment of national reimbursement rates, explains Lin.
“Although digital pathology, including usage of AI algorithms, could improve pathologist efficiency, this should not be the sole focus of reimbursement calculations. Digital pathology helps labs and pathologists expand their network of brainpower through greater access to information,
second opinions and subspecialist expertise. This ultimately could lead to optimized diagnostic decision-making and inherently leads to better patient management,” according to Esther Abels, Chief Clinical and Regulatory Officer, Visiopharm Corp. (Westminster, CO) and President of the Digital Pathology Association (Carmel, IN).