Volume Surge Expected For High-Priced Covid-19 Test Panels
With the flu season underway, more test panels are becoming available that test for Covid-19 plus other respiratory viruses, including influenza A/B and respiratory syncytial virus (RSV). Labs have begun to submit the combo PCR tests codes for Covid-19, although volume is nominal at this stage, notes Lale White, Chief Executive at XIFIN Inc. (San Diego, CA).
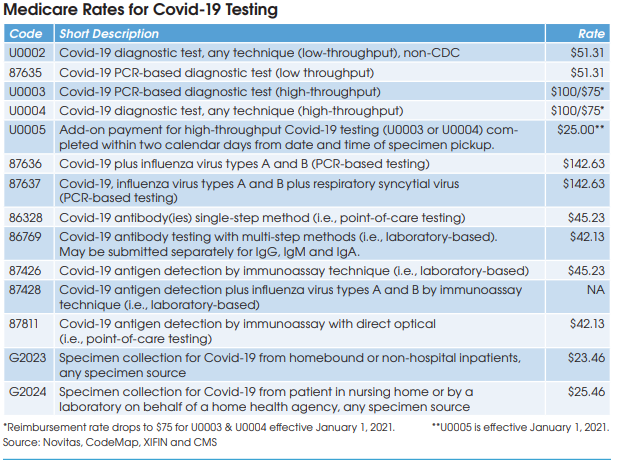
The AMA has issued new CPT codes for Covid-19 PCR-based test panels (87636 & 87637) and Medicare contractors have set rates for both of these codes at $142.63 by crosswalking to the existing code 87631 (respiratory virus detection, 3-5 targets).
In addition, several Proprietary Laboratory Analyses (PLA) codes (e.g., 0223U, 0202U and 0225U) have been issued for larger Covid-19 test panels that include up to 22 pathogen targets. Medicare contractors have set rates for these codes at $416.78 by crosswalking to the existing code
87507 (infectious agent detection, 12-25 targets).
There are currently an average of 1.5 million Covid-19 PCR tests being performed each day in the United States at cost of roughly $150 million per day, or $50+ billion annualized. A transition toward combo test panels reimbursed at $142-$417 per panel could potentially push the annualized market to more than $100 billion. That would exceed the total U.S. market for all non-Covid clinical lab and pathology testing.
In June 2020, the Office of Inspector General (OIG) communicated its fear that many labs are performing medically unnecessary add-on tests when responding to orders for Covid-19. The OIG has added an analysis for potential fraud and abuse with Covid-19 add-on testing to its work plan,
notes Charles Root, PhD, President of CodeMap LLC.