PAMA Reporting Period Delay Is Welcome News For Labs
On December 20, President Trump signed into law a spending package that included provisions of The LAB Act. The legislation delays the PAMA
private-payer data reporting schedule by one year, in order to give more time for all labs, especially hospital outreach labs, to gather and report data. The new schedule does not change the data collection period (Jan. 1 to June 30, 2019), but does delay the reporting of that data to CMS until Jan. 1 to March 31, 2021.
The reporting delay comes as a big relief to hospital outreach labs. Even the nation’s more sophisticated hospital outreach labs were planning to devote substantial billing and IT staff to meet the original deadline. The delay should allow more labs to report more complete and accurate payment data.
Furthermore, the delay will give ACLA a fighting chance to get something out of its PAMA lawsuit with CMS, notes lab industry consultant Dennis Weissman. “The original reporting schedule might have rendered the lawsuit moot. Now it has more time to move through the courts,” notes Weissman.
Julie Khani, President of the American Clinical Laboratory Assn., notes that there was broad bipartisan support for The LAB Act in both the House and Senate. In particular, she cites Rep. Scott Peters (D-CA), who introduced the House bill and raised The LAB Act with Energy & Commerce Committee Chair Frank Pallone (D-NJ) at a committee mark-up meeting earlier this year.
In addition to the delayed data reporting, The LAB Act directs the Medicare Payment Advisory Commission (MedPAC) to conduct a study to review how CMS has implemented the privatepayer-based Clinical Lab Fee Schedule (CLFS) under PAMA. As part of this study, MedPAC is to consider the least disruptive ways for CMS to collect data from labs and the most accurate and representative methods to determine payments rates, including the use of statistical methods for estimating rates that are representative of the whole lab market. MedPAC must report its findings to CMS and Congressional committees no later than 18 months from the enactment of The LAB Act (i.e., by late June 2021).
MedPAC is an independent U.S. federal body comprised of 17 members appointed by the Comptroller General of the United States. MedPAC’s Chair is Francis Crosson, MD, a former executive at the integrated managed care plan Kaiser Permanente. Its Vice Chair is Paul Ginsburg, PhD, a professor of health policy at the University of Southern California.
Of course there is no guarantee that MedPAC’s study recommendations will be favorable or that they will be acted upon by CMS or Congress.
Furthermore, using statistical sampling methods to capture a representative share of all sectors of the lab market (independents, hospital outreach and POLs) may be extremely complicated, notes Laboratory Economics. That’s because provider market share can vary widely for each of the 1,000+ CPT test codes on the CLFS.
Delayed Reporting Guarantees a Fourth Year of Cuts in 2021
The one-year delay in reporting means that CMS will continue to use pricing data from the initial PAMA survey to formulate CLFS rates for 2021. This guarantees rate cuts averaging 10-15% for most high-volume tests next year when the max reduction cap becomes 15%.
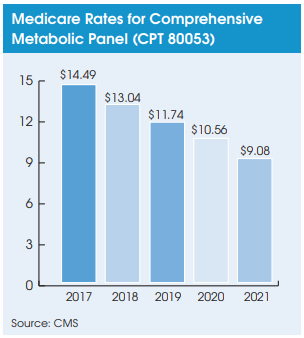
As highlighted in the last issue of Laboratory Economics, three straight years of 10% annual rate cuts (2018-2020) have not fully lowered many high-volume lab tests down to the median CLFS rates set by the initial PAMA survey. For example, after three years of the max 10% annual rate reduction, the comprehensive metabolic panel (CPT 80053) will still need another 14% cut next year to reach the median rate determined by the initial PAMA survey.
Any potential benefit from delayed PAMA reporting and the inclusion of more hospital outreach labs won’t occur until 2022.