PC Rates For Key Pathology Services To Get 12% Cut
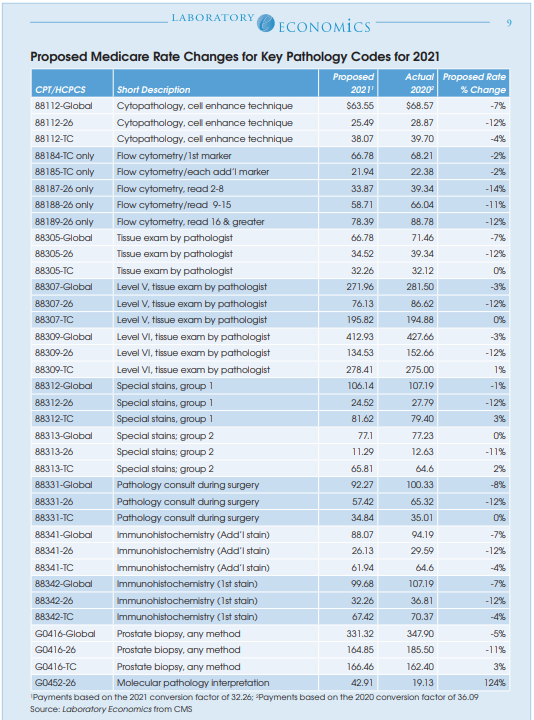
Medicare professional component (PC) reimbursement rates for most high-volume pathology services will be cut by 12% next year, according to the newly released Proposed Medicare Physician Fee Schedule (MPFS) for 2021. For example, the 2021 Medicare rate for the PC of CPT 88305 is proposed to be cut by 12% to $34.52, while the TC will remain the same at $32.26. Overall, the proposed global rate for CPT 88305 will decline by 7% to $66.78. The reductions are the result of budget neutrality requirements that offset the cost of major rate hikes given to evaluation and management (E/M) services paid to primary care physicians.
Overall, CMS estimates that the new rates will reduce Medicare reimbursement to pathologists by 9% in 2021, while technical component reimbursement to pathology labs will fall by 5%. Among the other specialties hurt by the redistribution of funds to primary care physicians include anesthesiology (-8%), emergency medicine (-6%), general surgery (-7%), infectious disease (-4%) and radiology (-11%). Specialties benefiting include endocrinology (+17%), family practice (+13%), hematology/oncology (+14%), nurse practitioner (+8%) and rheumatology (+16%).
Immunohistochemistry
The global rate for CPT 88342 (IHC, first stain procedure) is proposed to decrease by 7% to $99.68; professional interpretation down 12% to $32.26; technical component down 4% to $67.42.
The global rate for CPT 88341 (IHC, additional stain) is proposed to decline by 7% to $88.07; professional interpretation down 12% to $26.13; technical component down 4% to $61.94.
Molecular Pathology
One of the few bright spots in the proposed MPFS for 2021 is a recalculation of the rate for Molecular Pathology Interpretation (HCPCS code G0452). The current rate of $19.13 is proposed to more than double to $42.91 in 2021.
The Clinical Laboratory Fee Schedule An amendment (sec. 3718) to the CARES Act has further delayed the reporting period for labs to submit their private-payer payment data to CMS for the second PAMA survey cycle. Labs are still required to collect their private payer payment data from the period Jan. 1, 2019 through June 30,
2019, but the reporting period has been delayed to the first quarter of 2022. Medicare CLFS rates will be frozen in 2021, and lab test codes will then be subject to 15% max annual cuts from 2022 through 2024. CMS plans to finalize these changes when it issues its Final MPFS Rule this fall.